Atomic Model
Atomic Model: Overview
This Topic covers sub-topics such as Atom, Alpha Particles, Atomic Model, Drawbacks of Rutherford Model of Atom, Rutherford Model of Atom, Rutherford Experiment, The Thomson Model and, Brief History of Atomic Structure Upto Bohr Model
Important Questions on Atomic Model
The gravitational force between a -atom and another particle of mass will be given by Newton's law , where is in metre and
Geiger-Marsden experiment is related with the size of the-
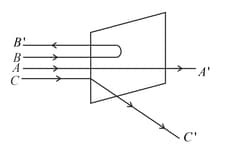
A beam of fast moving alpha particles were directed towards a thin film of Gold. The parts and of the transmitted and reflected beams corresponding to the incident parts and of the beam are shown in the adjoing diagram. The number of alpha particles in
In Rutherford's experiment, an -particle of mass and charge moves at high speed , along the -axis. It is initially near , and it ends up near The gold nucleus having charge is fixed at the point . As the -particle passes the stationary nucleus, its -component of velocity does not change appreciably, but it acquires a small velocity in the -direction. The angle through which the -particle is deflected is,
The total energy of an electron in the hydrogen atom is . The kinetic energy of the electron will be:
On which of the following factors does the trajectory of an alpha particle depend?
A standing wave in a string 35 cm long has a total of six nodes including those at the ends. Hence, wavelength of the standing wave is
Rutherford -scattering demonstrates the existence of which of the following ?
What is the conclusion of Rutherford’s alpha particle scattering experiment ?
-particles of energy are scattered back from a silver foil. If the maximum volume in which the entire positive charge of the atom is supposed to be concentrated is then find value of n. .
According to particle scattering experiment of Rutherford.
Here no. of α-particle scattered at an angle
Kinetic energy (initial) of particle.
What is the value of
Three particles having charges in the ratio of produce the same point on the photographic film in Thomson experiment. Their masses are in the ratio of
When a hydrogen atom is raised from ground energy level to excited energy level, then
Which of the following is incorrect regarding Rutherford's atomic model?
Which of the following properties of atom could be explained correctly by the Thomson Model of atom?
Which of the following is incorrect regarding Rutherford's atomic model?
